TAILORED FULL-SERVICE SOLUTIONS
Delivering customer-oriented, optimized evaluation services
through an integrated patient information model

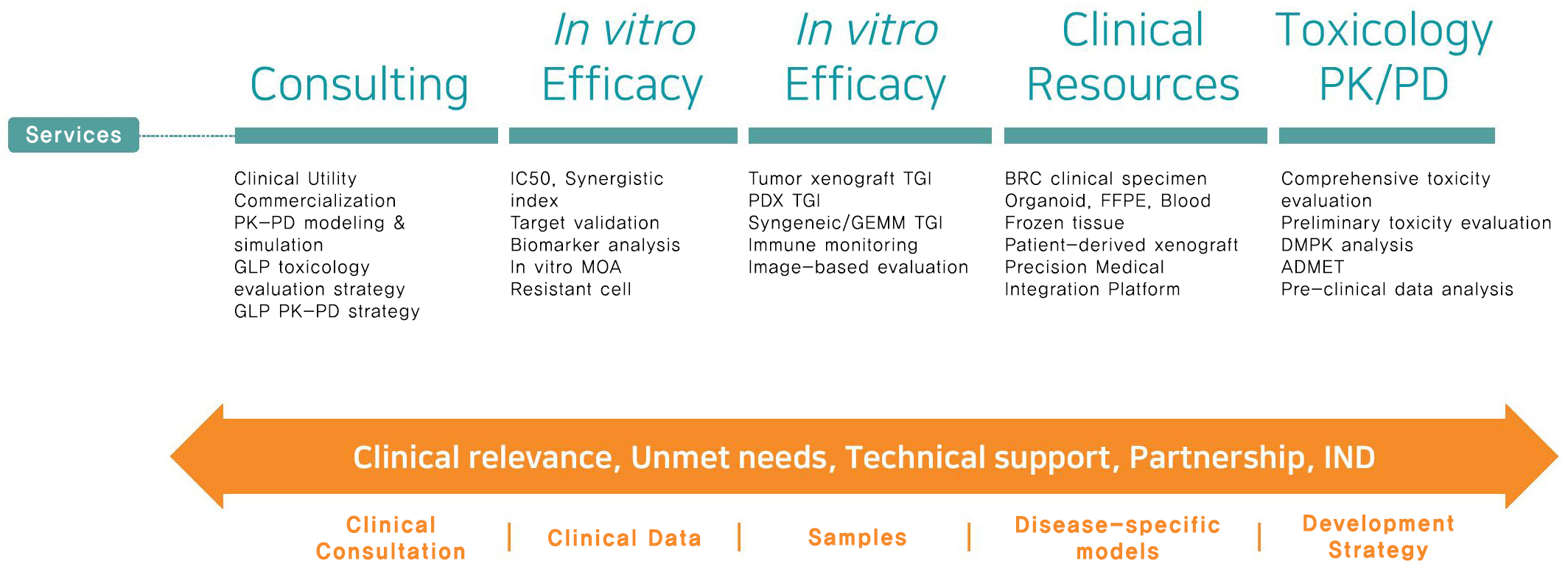
One-stop services for clinical and non clinical support

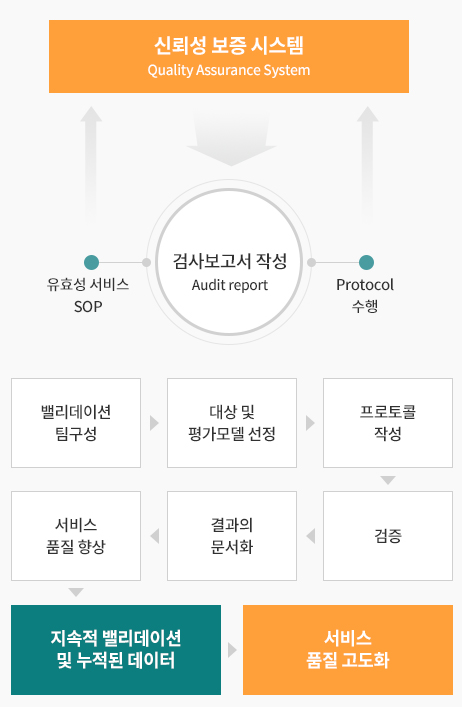
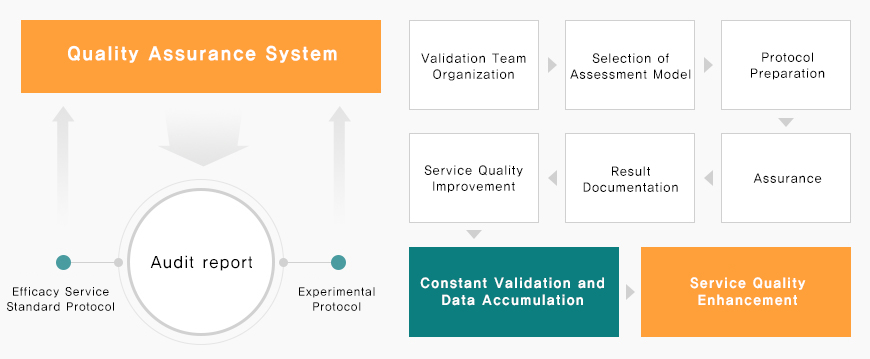
Quality Assurance System
At APEX, we implement written standard operating procedures (SOPs) to ensure consistent, high-quality services and efficient execution of all preclinical research activities.
Our Policy

Establish global regulatory documentation through strategic partnerships with our global clinical trial center and leading international institutions
Provide global coverage services by implementing the Common Technical Document (CTD) system

Maintain strict quality control and improvement through a robust quality enhancement system
Continuously monitor and improve service quality by validating each efficacy assessment method

Track project progress and share results in real time via our online project management system
Enable tailored analysis through real-time discussions

Operate QA activities under the direct supervision of the center director
Conduct SOP validation through QA inspections
Ensure all testing and documentation adhere to standardized protocols
Foster collaborative partnerships with clinical/preclinical CROs and advanced medical research complexes

Safeguard client intellectual property through Confidential Disclosure Agreements (CDA)
Enforce confidentiality through mandatory security training and individual confidentiality agreements for all PIs, PMs, researchers, and advisors
Use a secure platform