Assay systems for In vitro ADME profiling and PK studies using animal models
|
Mouse / Rat |
Analysis of drug concentrations in plasma or tissues after animal model development |
|
Rabbit / Dog |
Sample analysis (e.g., plasma) and calculation of pharmacokinetic (PK) parameters |
Service
- Bioanalysis
- Sample preparation and development of quantitative analysis methods using LC-MS/MS
- In vitro ADME assay
- Parallel artificial membrane permeability assay (PAMPA)
- Caco-2 permeability assay
- Protein binding assay
- Microsomal stability
- Plasma stability
- CYP inhibition study
- In vitro metabolite identification (Met ID)
- In vivo PK study
- Rodent (mouse/rat) full PK study
- Analysis of PK samples (e.g., plasma) from large animals and calculation of pharmacokinetic (PK) parameters
- Tissue distribution studies
- N-in-one PK study
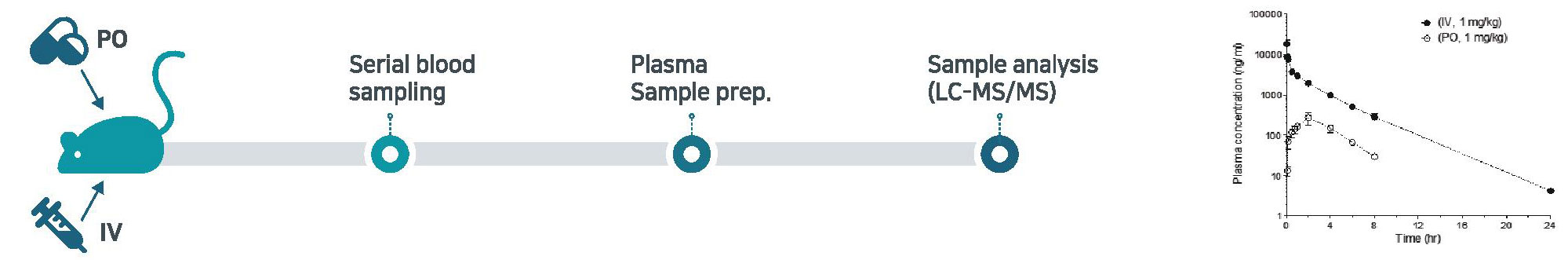
In vivo PK study
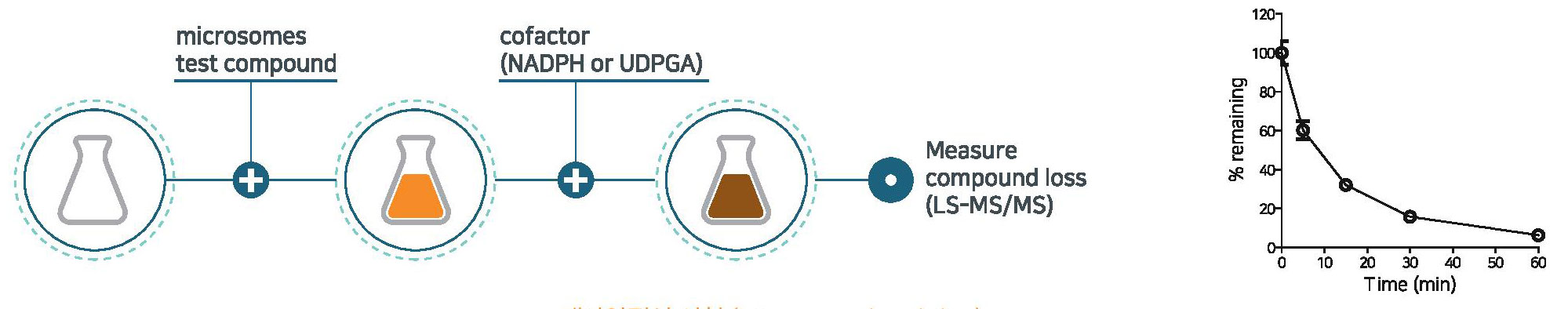
Microsomal stability
Service example
- PK studies in New Zealand White Rabbits
|
Design |
- Blood was collected from the veins of New Zealand White Rabbits at predefined time points following single-dose administration - Collected blood samples were transferred into sodium heparin tubes, then centrifuged at 4°C, 3000 rpm for 10 minutes to isolate plasma - Pharmacokinetic characteristics of the drug for animal models were determined based on the plasma drug concentration-time curve |
|
Result |

[Blood sampling time points] 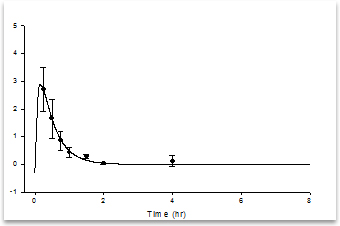
[Analysis result] |
Service example
- PK studies of oral administration in SD rat
|
Design |
- At each time point, 0.5 mL of blood was collected from the tail vein of male and female SD rats - Collected blood samples were transferred to sodium heparin tubes and centrifuged at 3,000 rpm for 10 minutes at 4°C to isolate plasma. - Pharmacokinetic characteristics of the drug for animal models were determined based on the plasma drug concentration-time curve |
|
Result |

[Blood sampling time points] 
[Analysis result] |





